
R&D Systems uses chemotaxis bioassays to measure the activity of the following chemokines and neutralizing antibodies:
Human Chemokines| 6Ckine | Fractalkine | MCP-3 | MPIF-1 |
| CCL28 | HCC-1 | MCP-4 | MPIF-2 |
| CTACK | I-309 | MDC | PARC |
| CXCR3 | I-TAC | MIG | RANTES |
| CXCR4 | IP-10 | MIP-1 alpha | SDF-1 alpha |
| Eotaxin | MCP-1 | MIP-1 beta | SDF-1 beta |
| Eotaxin-3 | MCP-2 | MIP-3 beta |
Mouse Chemokines
| 6Ckine | Eotaxin | MARC | MIP-3 beta |
| C10 | Fractalkine | MCP-5 | MPIF-2 |
| CCL28 | I-TAC | MIG | RANTES |
| CRG-2 | JE | MIP-1 alpha | SDF-1 alpha |
| CTACK | Lymphotactin | MIP-1 beta | TCA-3 |
Chemotaxis Bioassay MaterialsCell Preparation
|
Cell Growth and Preparation
As with all materials of human source, gloves and lab coats should be worn. All materials contaminated by these cells should be either decontaminated or disposed of in biohazard containers to be autoclaved. All procedures are carried out under sterile conditions.
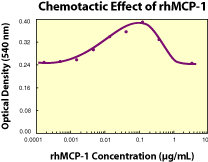 |
| Fig. 1. Human MCP-1 chemoattracts human monocytes, exhibiting a bell-shaped dose response curve. The ED50 for this effect is typically 0.005 - 0.02 µg/mL. |
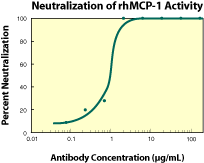 |
| Fig. 2. To measure the ability of the antibody to neutralize the chemoattractant activity of rhMCP-1 for human monocytes, rhMCP-1 was incubated with various concentrations of the antibody for 30 minutes at room temperature in a 96-well microplate. Following this preincubation period, 35 µL of the cytokine-antibody solution (containing rhMCP-1 at a final concentration of 0.1 µg/mL and antibody at the concentrations indicated) was transferred to the lower compartment of a 96-well chemotaxis chamber (NeuroProbe, Cabin John, MD). The chemotaxis chamber was then assembled using a PVP-free polycarbonate filter (8 micron pore size) and 1 x 106 cells/well was added to the top chamber. After incubation for 75 minutes at 37 °C in a 5% CO2 humidified incubator, the chamber was disassembled and the filter was fixed and stained using Leukostat (Fisher Scientific). The optical density of the filter, which is proportional to the number of cells that migrated across the filter, was then read in a microplate reader set at 540 nm. As shown in figure 2, the ND50 for this lot of antibody is approximately 0.8 - 2.5 µg/mL. |
R&D Systems uses HUVECs (Human Umbilical Vein Endothelial Cells) in proliferation bioassays of the following cytokines and neutralizing antibodies:
HUVEC Bioassay MaterialsCell Growth and Preparation
|
Cell Growth and Preparation
As with all materials of human source, gloves and lab coats should be worn. All materials contaminated by these cells should be either decontaminated or disposed of in biohazard containers to be autoclaved. All procedures are carried out under sterile conditions.
 |
| Figure 1. Human VEGF stimulates the 3H-thymidine incorporation by human umbilical vein endothelial cells in a dose-dependent manner. The ED50 for this effect is typically 2-6 ng/mL. |
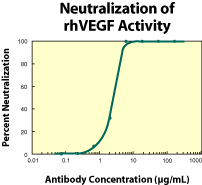 |
| Figure 2. To measure the ability of the antibody to neutralize the bioactivity of rhVEGF on human umbilical vein endothelial cells, rhVEGF was incubated with various concentrations of the antibody for 1 hour at 37 °C in a 96-well microplate. Following this preincubation period, HUVECs were added. The assay mixture in a total volume of 100 µL, containing antibody at the concentrations indicated, rhVEGF at 10 ng/mL and cells at 0.5 x 104 cells/well, was incubated at 37 °C for 72 hours in a humidified CO2 incubator. 3H-thymidine was added during the final 24 hours of incubation. The cells were subsequently harvested onto glass fiber filters and the 3H-thymidine incorporated into DNA was determined. The ND50 of the antibody is approximately 3-6 µg/mL. |
R&D Systems uses NR6R-3T3 cells in proliferation bioassays to measure the activity of the following cytokines and neutralizing antibodies:
NR6R-3T3 Cell Bioassay Materials |
Cell Growth and Preparation
|
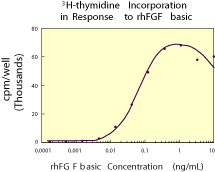 |
| Figure 1. Human FGF basic stimulates the 3H-thymidine incorporation by NR6R-3T3 fibroblasts in a dose-dependent manner.1 The ED50 for this effect is typically 0.1 - 0.25 ng/mL. |
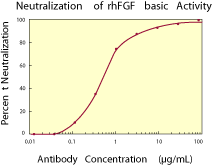 |
| Figure 2. To measure the ability of the antibody to neutralize the bioactivity of rhFGF basic on NR6R-3T3 fibroblasts, human FGF basic was incubated with various concentrations of the antibody for 1 hour at 37 °C in a 96-well microtiter plate. Following this preincubation period, the antigen-antibody mixture was added to quiescent confluent cultures of NR6R-3T3 cells in DMEM with 2% bovine plasma-derived serum. The assay mixture in a total volume of 100 µL, containing antibody at the concentrations indicated and rhFGF basic at 0.5 ng/mL, was incubated at 37 °C for 20 hours in a humidified CO2 incubator. 3H-thymidine was added during the final 2 hours of incubation. The cells were subsequently detached and harvested onto glass fiber filters and the 3H-thymidine incorporated into DNA was determined. The ND50 of the antibody is approximately 0.5 - 1.0 µg/mL. |
Cell Growth and Preparation
As with all materials of human source, gloves and lab coats should be worn. All materials contaminated by these cells should be either decontaminated or disposed of in biohazard containers to be autoclaved. All procedures are carried out under sterile conditions.
Doubling Time: Approximately 17 hours Appearance: Rectangular shaped, attached cells
TF-1 cells are a factor-dependent human erythroleukemic cell line. TF-1 cells are employed in proliferation bioassays by R&D Systems for the routine quality assurance and quality control of the following cytokines and neutralizing antibodies:
TF-1 cells will also proliferate in response to human IL-6 and IL-11. TF-1 cells are not responsive to human G-CSF, IL-2, IL-7, IL-9 and M-CSF. The TF-1 cells used by R&D Systems were obtained from Dr. Toshio Kitamura in 1989. Dr. Kitamura has deposited TF-1 cells with ATCC (#CRL-2003). The TF-1 cells deposited by Dr. Kitamura with the ATCC are described as non-responsive to IL-5. If you need to obtain TF-1 cells that will respond to IL-5, please contact R&D Systems Technical Service at 1-800-343-7475.
Cell Growth and Preparation
TF-1 Bioassay
|
As with all materials of human source, gloves and lab coat should be worn. All materials contaminated by these cells should be either decontaminated or disposed of in biohazard containers to be autoclaved. All procedures are carried out under sterile conditions.
Doubling Time: Approximately 30 hours.
Appearance: Single cells, slightly irregular in size and shape. A small percentage will attach to the flask.
 |
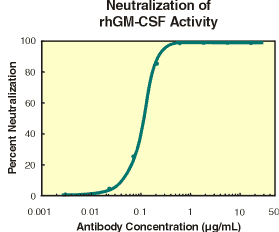 |
| Figure 1. Human GM-CSF stimulates the 3H-thymidine incorporation by TF-1 cells in a dose-dependent manner (Kitamura, T. et al., (1989) J. Cell Physiol. 140(2):323). The ED50 for this effect is typically 0.02 - 0.08 ng/mL. | Figure 2. Typical data for anti-hGM-CSF (Catalog # AF-215-NA) is shown. To measure the ability of the antibody to neutralize the bioactivity of rhGM-CSF on human TF-1 cells, rhGM-CSF was incubated with various concentrations of the antibody for 1 hour at 37° C in a 96-well plate. Following this preincubation period, TF-1 cells were added. The assay mixture in a total volume of 100 µL, containing antibody at the concentrations indicated, rhGM-CSF at 0.5 ng/mL and cells at 1 x 105cells/mL, was incubated at 37° C for 48 hours in a humidified CO2 incubator.3H-thymidine was added during the final 4 hours of incubation. The cells were subsequently harvested onto glass fiber filters and the 3H-thymidine incorporated into DNA was determined. The ND50 of the antibody is approximately 0.08 - 0.16 µg/mL. |