Why Verify the Multipotency of Neural Progenitor Cells?
Confirmation of multipotency is a critical experimental step when studying neural progenitor cells (NPCs). The kit and protocol presented here provide a quick and straightforward technique to confirm the ability of your starting cell population to differentiate toward astrocyte, neuron, and oligodendrocyte lineages. Neural stem cell research studies most often require weeks of cell culture before an experimental hypothesis can be tested. The ability to verify multipotency at an early stage in the experimental design provides valuable insight that may prevent weeks of wasted effort and reagents. Moreover, confirmation of multipotency helps to ensure consistency among studies and reduce unwanted experimental variability.
This protocol is designed to assess the multipotency of neural progenitor cells using the Human/Mouse/Rat Neural Lineage Functional Identification Kit (Catalog # SC028).
Materials Supplied in the Human/Mouse/Rat Neural Lineage Functional Identification Kit (Catalog # SC028)
-
Neural Maintenance Supplement
-
Neural Differentiation Supplement
-
Bovine Fibronectin 100X
-
Goat Anti-Rat Nestin Antigen Affinity-purified Polyclonal Antibody
-
Sheep Anti-Human GFAP Antigen Affinity-purified Polyclonal Antibody
-
Mouse Anti-Neuron-Specific beta-III Tubulin Monoclonal Antibody
-
Mouse Anti-Oligodendrocyte Marker O4 Monoclonal Antibody
OTHER SUPPLIES REQUIRED
Materials
-
Human, mouse, or rat neural progenitor cells
-
24-well culture plates
-
12 mm cover slips
-
15 mL centrifuge tubes
-
Pipettes and pipette tips
-
Serological pipettes
-
Fine pointed curved forceps
-
Glass slides
-
Slide box
Reagents
-
N-2 MAX Media Supplement (Catalog # AR009)
-
Poly-L-ornithine
-
Phosphate Buffered Saline (PBS)
-
Penicillin-Streptomycin 100X
-
Bovine Serum Albumin (BSA)
-
DMEM/F12
-
Glucose
-
L-Glutamine
-
Sodium Bicarbonate (NaHCO3)
-
Trypan blue
-
95% Ethanol
-
4% Paraformaldehyde in PBS
-
1% BSA in PBS
-
1% BSA, 10% normal donkey serum in PBS
-
0.3% Triton® X-100, 1% BSA, 10% normal donkey serum in PBS
-
Mounting medium (Catalog # CTS011)
-
Secondary antibody (Catalog # NL001, NL007, NL010, NL019, or equivalent)
-
DAPI (4',6-diamidino-2-phenylindole) solution: Add 1 μL of 14.3 mM stock for every 5 mL of PBS. Store any unused DAPI at 2 °C - 8 °C, wrapped in aluminum foil
-
Deionized or distilled water
Equipment
-
37 °C and 5% CO2 incubator
-
Centrifuge
-
Hemocytometer
-
Inverted microscope
-
37 °C water bath
-
Fluorescence microscope
-
0.2 μm filter unit, 250 mL
REAGENT AND MATERIAL PREPARATION
Use serological pipettes to transfer and remove solutions.
Preparation of Completed Base Media
Completed Base Media - Mix the components listed in the table below with deionized or distilled water to make 200 mL of Completed Base Media. Adjust the pH to 7.2 ± 0.2. Sterile filter the solution using a 0.2 μm filter unit, and store in the dark at 2-8 °C for up to 2 weeks.
|
Component |
Amount |
|
DMEM-F12 |
2.4 g |
|
Glucose |
0.31 g |
|
L-Glutamine |
0.0146 g |
|
NaHCO3 |
0.338 g |
|
N-2 MAX Media Supplement |
2 mL |
|
Note: If desired, Penicillin-Streptomycin can be added to a final concentration of 1X. |
Preparation of 24-Well Plate
Poly-L-ornithine (1000X) - Dissolve Poly-L-ornithine in sterile PBS to make a 15 mg/mL stock. Aliquot and store at < -20 °C in a manual defrost freezer for up to 6 months. Avoid repeated freeze-thaw cycles.
Poly-L-ornithine Solution (1X) - Dilute 1000X Poly-L-ornithine 1000-fold in sterile PBS to make a 1X solution (15 μg/mL). Prepare fresh as needed.
Bovine Fibronectin Solution (1X) - Dilute the 100X Bovine Fibronectin 100-fold in sterile PBS to make a 1X solution (1 μg/mL). Mix by gentle swirling, without vortexing. Prepare fresh as needed.
Preparation of Lyophilized Antibodies
Goat Anti-Rat Nestin (10X) - Reconstitute with 500 μL of sterile PBS. Mix gently. Aliquot and store at < -20 °C in a manual defrost freezer for up to 6 months. Avoid repeated freeze-thaw cycles.
Sheep Anti-Human GFAP (10X) - Reconstitute with 500 μL of sterile PBS. Mix gently. Aliquot and store at < -20 °C in a manual defrost freezer for up to 6 months. Avoid repeated freeze-thaw cycles.
Mouse Anti-Neuron-specific beta-III Tubulin (10X) - Reconstitute with 500 μL of sterile PBS. Mix gently. Aliquot and store at < -20 °C in a manual defrost freezer for up to 6 months. Avoid repeated freeze-thaw cycles.
Mouse Anti-Oligodendrocyte Marker O4 (10X) - Reconstitute with 500 μL of sterile PBS. Mix gently. Aliquot and store at < -20 °C in a manual defrost freezer for up to 6 months. Avoid repeated freeze-thaw cycles.
PROCEDURES
-
Preparation of Poly-L-ornithine and Fibronectin Coated Plates
-
Insert a sterile cover slip (sterilized with 95% EtOH and flamed) into each well of a 24-well plate.
-
Add 0.5 mL of Poly-L-ornithine Solution (1X) to each well. Gently sink the floating cover slips with a sterile pipette tip. Incubate overnight at 37 °C.
-
Discard the Poly-L-ornithine Solution. Wash each well 3 times with 1 mL of sterile PBS each time.
-
Add 0.5 mL of sterile PBS to each well. Incubate overnight at 37 °C.
-
Discard the PBS. Wash each well once with 1 mL of sterile PBS.
-
Add 0.5 mL of 1X Fibronectin solution to each well. Gently sink the floating cover slips with a sterile pipette tip.
-
Incubate in a 37 °C incubator for 3-24 hours.
-
Discard the 1X Fibronectin Solution and wash each well once with 1 mL of PBS before proceeding to the Cell Plating protocol.
-
Cell Plating and Maintenance
Fresh supplemented media should be made for each usage or media change. The recommended amount of media for a 24-well plate is 0.5 mL/well. Make 12 mL of media for 24 wells.
-
Add 24 μL of the Neural Maintenance Supplement to 12 mL of Completed Base Media. Mix gently.
-
Seed 0.5 - 1.0 x 106 NPCs in 12 mL of Completed Base Media containing the Neural Maintenance Supplement on a Poly-L-ornithine/Fibronectin Coated Plate at 0.5 mL/well.
-
Incubate the cells at 37 °C and 5% CO2. Cells should become adherent after 24 hours.
-
Twenty-four hours after the initial plating, replace the media with fresh Completed NSC Base Media containing the Neural Maintenance Supplement.
-
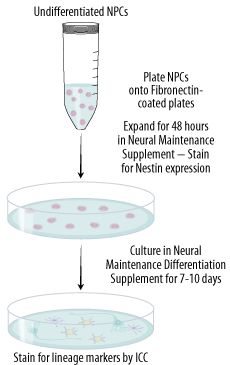
Forty-eight hours after the initial plating, cells from two wells can be evaluated by immunocytochemistry for Nestin expression (step IV) and the rest of the wells are ready for differentiation (step III).
-
Cell Differentiation
Differentiation of NPCs is performed 48 hours after the initial cell plating and culturing in Completed Base Media containing Neural Maintenance Supplement.
Fresh supplemented media should be made for each usage or media change (12 mL of media is required for each media change).
-
Add 120 μL of the Neural Differentiation Supplement to 12 mL of Completed Base Media. Mix gently.
-
Remove the media from the wells and wash once with sterile PBS.
-
Add 0.5 mL of Completed Base Media containing the Neural Differentiation Supplement to each well.
-
Replace the media with fresh Completed Base Media containing the Neural Differentiation Supplement every three days.
-
Cells can be fixed for characterization after seven days of differentiation in Completed Base Media containing the Neural Differentiation Supplement.
-
Characterization of Cells by Immunocytochemistry
Nestin, Neuron-specific beta-III Tubulin and GFAP are used as markers for neural stem cells, neurons, and astrocytes, respectively.
Note: A negative control should be performed using PBS containing 1% BSA and 10% normal donkey serum with no primary antibody.
Note: DAPI counterstain can obscure visualization of targets localized in cell nuclei.
-
Remove the media from wells selected for immunocytochemistry characterization and wash the cells twice with 1 mL of PBS.
-
Fix the cells with 0.5 mL of 4% paraformaldehyde in PBS for 20 minutes at room temperature.
-
Wash the cells three times with 0.5 mL of 1% BSA in PBS for 5 minutes each.
-
Permeabilize and block cells with 0.5 mL of 0.3% Triton X-100, 1% BSA, and 10% normal donkey serum in PBS at room temperature for 45 minutes.
-
During step 4, when cells are being blocked, dilute the reconstituted Goat Anti-Rat Nestin, Mouse Anti-Neuron-specific beta-III Tubulin, or Sheep Anti-Human GFAP antibody in PBS containing 1% BSA and 10% normal donkey serum to the suggested final concentration.
-
After blocking, incubate the cells with 300 μL/well of 1X Goat Anti-Rat Nestin, Mouse Anti-Neuron-specific beta-III Tubulin or Sheep Anti-Human GFAP overnight at 2 °C - 8 °C.
-
Wash the cells three times with 0.5 mL of 1% BSA in PBS for 5 minutes each.
-
Dilute the appropriate secondary antibody (e.g. NL557-conjugated donkey anti-mouse IgG, donkey anti-goat IgG, or donkey anti-sheep IgG secondary antibody, Catalog # NL001, NL007, NL010) at 1:200 in PBS containing 1% BSA.
-
Incubate the cells with 300 μL/well of secondary antibody in the dark at room temperature for 60 minutes.
-
Wash the cells three times with 0.5 mL of 1% BSA in PBS for 5 minutes each.
-
Wash the cells once with 0.5 mL of PBS for 5 minutes.
-
Add 300 μL of the diluted DAPI solution to each well, and incubate 2-5 minutes at room temperature. DAPI binds to DNA and is a convenient nuclear counterstain. It has an absorption maximum at 358 nm and fluoresces blue at an emission maximum of 461 nm.
-
Wash the cells once with 0.5 mL of PBS for 5 minutes.
-
Aspirate the PBS from the wells and add 0.5 mL of deionized or distilled water. Carefully remove the cover slips with forceps and mount cell side down onto a drop of mounting media on a glass slide.
-
The slides are ready for microscopic observation.
-
Immunocytochemistry of Oligodendrocytes with Mouse Anti-Oligodendrocyte Marker O4
Note: A negative control should be performed using PBS containing 1% BSA and 10% normal donkey serum with no primary antibody.
Note: DAPI counterstain can obscure visualization of targets localized in cell nuclei.
-
Remove the media from wells selected for immunocytochemistry characterization and wash the cells twice with 1 mL of PBS.
-
Fix the cells with 0.5 mL of 4% paraformaldehyde in PBS for 20 minutes at room temperature.
-
Wash the cells three times with 0.5 mL of 1% BSA in PBS for 5 minutes each.
-
Block the cells with 0.5 mL of 1% BSA and 10% normal donkey serum in PBS at room temperature for 45 minutes.
-
During step 4 when the cells are being blocked, dilute the reconstituted Mouse Anti-Oligodendrocyte Marker O4 antibody in PBS containing 1% BSA and 10% normal donkey serum to a final concentration of 0.2 μg/100 μL.
-
After blocking, incubate the cells with 300 μL/well of Mouse Anti-Oligodendrocyte Marker O4 overnight at 2 °C - 8 °C.
-
Wash the cells three times with 0.5 mL of 1% BSA in PBS for 5 minutes each.
-
Dilute the secondary antibody (e.g. NL557-conjugated Donkey Anti-Mouse IgM Secondary Antibody, Catalog # NL007) at 1:200 in PBS containing 1% BSA.
-
Incubate the cells with 300 μL/well secondary antibody in the dark at room temperature for 60 minutes.
-
Wash the cells three times with 0.5 mL of 1% BSA in PBS for 5 minutes each.
-
Add 300 µL of the diluted DAPI solution to each well, and incubate 2-5 minutes at room temperature. DAPI binds to DNA and is a convenient nuclear counterstain. It has an absorption maximum at 358 nm and fluoresces blue at an emission maximum of 461 nm.
-
Wash the cells once with 0.5 mL of PBS for 5 minutes.
-
Aspirate the PBS from the wells and add 0.5 mL of deionized or distilled water. Carefully remove the cover slips with forceps and mount cell side down onto a drop of mounting media (Catalog # CTS011) on a glass slide.
-
The slides are ready for microscopic observation.
Data Examples
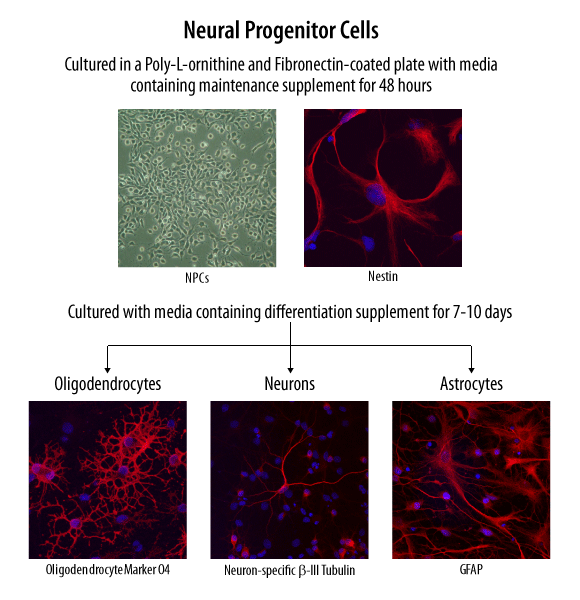
Verification of Neural Progenitor Cell Multipotency. Neural progenitor cells were maintained in culture and differentiated towards neural lineages using the specialized media supplements supplied in the Human/Mouse/Rat Neural Lineage Functional Identification Kit (Catalog # SC028). Neural progenitor cell multipotency was functionally verified using an antibody panel to detect phenotype-specific markers for neural precursors, astrocytes, neurons, and oligodendrocytes (also supplied in the kit).